The Challenge of Transfusion Safety and Occult Hepatitis B (OBI)
The usually accepted definition of Occult Hepatitis B (OBI) infection is an infection not detectable by a Hepatitis B (HBV) surface antigen test (HBsAg) but detectable by molecular testing of DNA in serum or liver biopsy. Because of its sporadic detectability even by state-of-the-art serology tests and very low viral load in serum and plasma, HBV remains the largest viral threat for transfusion transmission even after serology and NAT testing of donated blood. We will discuss why OBI is difficult to detect and how testing protocols differ around the world.
Hepatitis B Detection
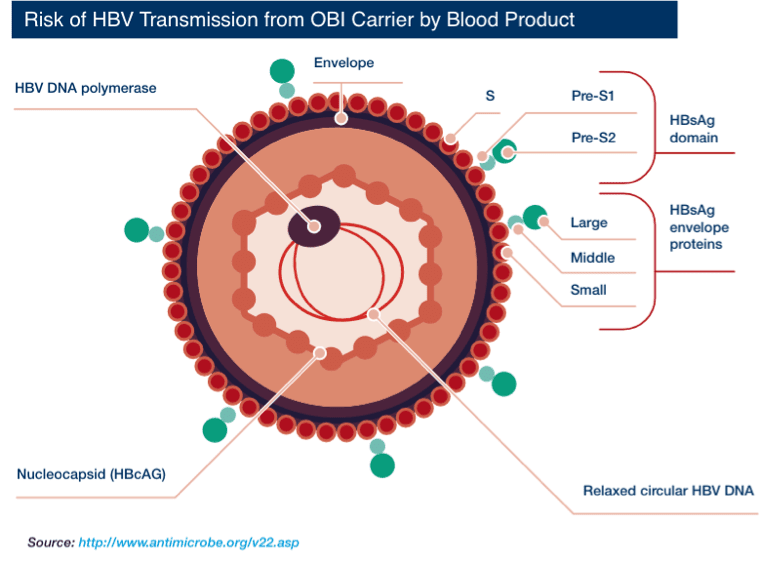
Hepatitis B virus (HBV) is an enveloped virus. Each virion (virus particle or Dane particle) is 42 nm in diameter. The three surface proteins of HBV — S (small), pre-S2 (middle), and pre-S1 (large) — can also form viral subparticles as rods and spheres. These viral subparticles are more abundant than Dane particles ("parent" virions).
Serologic (antibody) and DNA markers appear at different times during the clinical course of HBV infection. HBV DNA can be detected by nucleic acid testing (NAT) as early as 15 days after infection. This early marker of infection is followed by hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg), which is an indicator of infectivity. In the case of chronic (long-term) HBV infection, antibodies against HBsAg, HBeAg, and core antigen (anti-HBc) can be detected for years after infection. Because of occult HBV infection (OBI) in donors, HBV remains the most frequent viral infection after blood transfusion [Esposito et al. 2017].
Because of occult HBV infection (OBI) in donors, HBV remains the most frequent viral infection from blood transfusion.
What is Occult Hepatitis B Infection (OBI)?
OBI was first described in the 1970s in chronically ill patients with liver disease; that is, HBV DNA was detected, but HBsAg was negative.
At an international workshop in Taormina, Italy, in 2008, OBI was defined by the European Association for the Study of the Liver as [Esposito et al. 2017; Raimondo et al. 2008]:
"The presence of HBV DNA in the liver, with detectable or undetectable HBV DNA in the bloodstream, of individuals testing negative for HBsAg by currently available assays."
However, for practical purposes, OBI is often defined as the presence of serum HBV DNA without the detection of HBsAg. Patients with OBI might be seropositive for anti-HBs, anti-HBc, or both.
OBI is often defined as the presence of serum HBV DNA without the detection of HBsAg.
Prevalence of OBI
The prevalence of OBI varies with HBV prevalence, population characteristics, and the sensitivity of assays used for detection. Various geographic areas can be classed according to HBV prevalence [Esposito et al. 2017]:
- High endemicity (prevalence ≥8%) — the Amazon Basin, China, Southeast Asia, and Sub-Saharan Africa
- Intermediate endemicity (prevalence 2–8%) — Eastern Europe, Mediterranean regions, and the Middle East
- Low endemicity (prevalence <2%) — Australasia, Northern and Western Europe, North and South America
Serum levels of HBV DNA in patients with OBI are generally low. For instance, cases have been reported of repeat donors who were HBsAg-negative, but anti-HBs and anti-HBc positive, with DNA concentrations ranging from 8–260 IU/mL over 7 years. As such, low viral loads can sometimes be close to the NAT assay detection limit (≈5–10 IU/mL), several countries recommend supplemental serologic testing for anti-HBc and other viral markers (see below) [Esposito et al. 2017].
Data strongly indicate that host responses, including immune responses and epigenetic factors (ie, covalent modifications of DNA or histone proteins, and post-transcriptional changes) have a major role in influencing HBV activity. Coinfection from HBV and other organisms may also be a potential mechanism of OBI. Conversely, viral genomic variability does not seem to have a fundamental role in OBI status [Kwak and Kim 2014].
Infectivity of OBI Through Blood Donation
Blood donors with OBI can transmit HBV via blood donations and organ transplantation. The infectivity of OBI depends on whether [Allain et al. 2013; Esposito et al. 2017]:
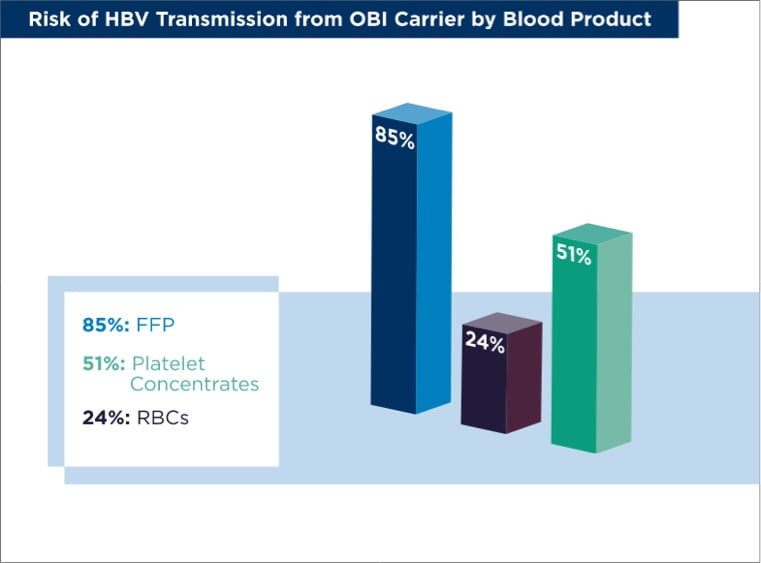
- The transfused product is platelet concentrate, red blood cells (RBCs), or fresh frozen plasma (FFP). In these cases, viral dose and transfused plasma volume are the prime factors influencing HBV transmission. The risk of HBV transmission to unvaccinated patients from an anti-HBs-negative OBI carrier is 24% for RBCs, 51% for platelet concentrates, and 85% for FFP.
- The donor is anti-HBs+. Donors who are anti-HBs+/anti-HBc+ may have a five-fold reduction in the risk of HBV transmission to recipients. Further, HBV transmission is unlikely from OBI carriers with high levels of anti-HBs, although transmission may occur from carriers who donate "anti-HBc only" blood products. In fact, in regions of high HBV endemicity, most transfusion recipients have already had HBV infection; therefore, the post-transfusion risk of infection is generally low, although it is high in individuals previously uninfected with HBV. Typically, a donor anti-HBs level <100 IU/L provides only limited protection against the usually low risk of HBV DNA transmission. In much of the world, HBV vaccination is lowering transmission risk as well.
- The recipient is immunocompetent (ie, has a normally functioning immune system).
How to Detect Occult Hepatitis B Infection
NAT is effective for OBI detection. It permits the daily processing and release of numerous blood products, including short half-life components such as platelet concentrates. However, for NAT detection of HBV, two key factors require emphasis and re-emphasis [Esposito et al. 2017]:
- NAT detection of human immunodeficiency virus or hepatitis C virus is generally more effective than NAT for HBV. This is because HBV replication is slow: the time for viral load doubling is ≈2.6 days, even during the early stages of infection.
- In patients with OBI, the amount of HBV DNA present are very low.
Individual donation (ID)-NAT testing can reach a sensitivity of a few IU/mL and shows sensitivity advantages over pool or mini-pool (MP) testing for OBI detection [Esposito et al. 2017]. For example, during 4 years of ID-NAT in South Africa, and in which almost 3 million blood donors were screened, 177 cases of OBI were identified. Among these cases, 75 were of the anti-HBc+/anti-HBs– variety and were potentially infectious [Vermeulen et al. 2012]. Generally, in regions of low HBV endemicity (eg, Australasia), MP-NAT for HBV is appropriate; conversely, in regions of high HBV endemicity (eg, China), ID-NAT should be used to enhance blood-supply safety [Esposito et al. 2017].
To ensure high HBV detection rates, some blood establishments adopt a repeat-testing strategy. For example, in South Africa, additional duplicate NAT tests plus the discriminatory tests are performed after an initial reactive result is identified. Samples that are repeat-reactive for both NAT and serologic assays are classified as confirmed infections. The donors are then notified and deferred [Vermeulen et al. 2012]. Donations that are repeat-reactive on NAT or discriminatory testing, but negative on serologic testing, are classified as potential NAT yields. These donors are contacted and a follow-up sample is requested for repeat testing.
Comparing Testing Methods for Anti-HBc
In a Polish study of approximately 1 million donors, 28 HBV DNA+/HBsAg– donors were identified. Most of these donors (n=21) were anti-HBc+, and the donors were either anti-HBs+ (n=5) or anti-HBs– (n=23) [Brojer et al. 2006]. More recently, in a US study, 22.4 million blood donations underwent NAT for HBV DNA and serologic testing for anti-HBc and HBsAg. Overall, the rate of OBI was 1.81 per 100,000 person-years. Among the 404 OBI samples identified, most (89%) were detected by ID-NAT, whereas the remainder (11%) were detected only by MP-NAT, suggesting that most donors with OBI had HBV DNA levels <5–10 copies/mL [Dodd et al. 2018].
As already mentioned, viral loads in OBI samples can be close to the NAT assay detection limit. This means that some blood donors who test negative for HBV DNA and HBsAg, but who show reactivity for anti-HBc, may carry an HBV viral load below the NAT assay detection limit. Therefore, anti-HBc is recognized as a major indicator of OBI, and anti-HBc testing is routinely used in several geographic areas as one of the standard serologic tests for blood donors, especially HBsAg– donors. However, anti-HBc testing is contentious in some regions because of adverse cost-benefit considerations and low specificity (a high false-positive rate), which leads to unnecessary deferral of donors. Anti-HBc testing is of limited value in areas of high HBV endemicity (eg, Sub-Saharan Africa) [Esposito et al. 2017].
In countries with a low prevalence of HBV, such as Canada and the US, anti-HBc screening is used in the donor screening process, in addition to NAT. Anti-HBs levels >100 IU/L provide adequate protection against transfusion-transmitted infections, even though blood is anti-HBc+. Therefore, a blood-screening strategy that monitors anti-HBs and anti-HBc levels, together with NAT, is used by many countries. However, for countries with an intermediate or high prevalence of HBV, screening for anti-HBc might compromise national blood supplies due to the higher proportions of anti-HBc+ donors.
Summary of OBI Challenges and Solutions
- OBI is a significant factor regarding HBV transfusion-transmission risk. It is a major concern, in regions with high HBV endemicity, for patients undergoing blood transfusion or organ transplantation.
- NAT plays a vital role in OBI detection and in reducing the risk of transfusion-transmitted HBV infection.
- Overall, blood-testing strategies and algorithms for HBV detection, and the cost-benefit ratios of blood-testing procedures, need to be evaluated according to HBV prevalence data for specific countries and blood establishments.
References
- Esposito A, Sabia C, Iannone C, et al. Occult hepatitis infection in transfusion medicine: screening policy and assessment of current use of anti-HBc testing. Transfus Med Hemother 2017;44:263–72.
- Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008;49:652–7.
- Kwak MS, Kim YJ. Occult hepatitis B virus infection. World J Hepatol 2014;6:860–9.
- Allain JP, Mihaljevic I, Gonzalez-Fraile MI, et al. Infectivity of blood products from donors with occult hepatitis B virus infection. Transfusion 2013;53:1405–15.
- Brojer E, Grabarczyk P, Liszewski G, et al. Characterization of HBV DNA+/HBsAg– blood donors in Poland identified by triplex NAT. Hepatology 2006;44:1666–74.
- Dodd RY, Nguyen ML, Krysztof DE, et al. Blood donor testing for hepatitis B virus in the United States: is there a case for continuation of hepatitis B surface antigen detection? Transfusion 2018;58:2166–70.
- Vermeulen M, Dickens C, Lelie N, et al. Hepatitis B virus transmission by blood transfusion during 4 years of individual-donation nucleic acid testing in South Africa: estimated and observed window period risk. Transfusion 2012;52:880–92.