Antiphospholipid Syndrome (APS) Diagnostics

Antiphospholipid syndrome (APS) is a systemic autoimmune disease that increases the risk of blood clots. Patients with APS are at risk of vascular complications (thrombotic APS) and, for women, pregnancy-associated complications (obstetric APS). The primary vascular complications are deep vein thrombosis, arterial thrombosis (which can cause a stroke or heart attack), and stroke (blood clots in the brain). This topic will examine key diagnostics for antiphospholipid syndrome.
Symptoms of Antiphospholipid Syndrome
Symptoms of thrombosis, or blood clots, indicate APS. Although, many patients do not have any symptoms of thrombosis, just tissue damage that develops from blood clotting significantly reducing blood flow to a particular organ. If the clots affect the brain, patients may experience partial paralysis, speech loss, dementia, and seizures, but minor strokes or transient ischemic attacks may not cause permanent damage. Heart damage may occur from a heart attack, or from damage to valves in the heart or veins.
Deep vein thrombosis may be associated with pain, swelling, and redness in the legs. Some people can develop a rash and, less commonly, bleeding complications. If a clot dislodges it may travel to the lungs and cause a pulmonary embolism, which can have serious consequences (including sudden death). Microangiopathy is another vascular complication, which occurs when clots affect small blood vessels. It may result in renal thrombosis if it involves the kidneys, cardiomyopathy if it involves the heart, and ulcers, necrosis, and livedo reticularis (patterned discoloration of the skin) if it involves the skin.¹
Pregnancy complications of obstetric APS can include ²⁻³:
- Recurrent spontaneous miscarriages or stillbirths
- Intrauterine growth retardation
- Preeclampsia or pregnancy-related high blood pressure
In rare circumstances, patients with APS can develop multi-organ thrombosis. This life-threatening condition is called "catastrophic APS." For people who already have a diagnosis of APS, discontinuation of anticoagulant therapy could trigger catastrophic APS. Other triggers include infections and surgical procedures.
Common non-thrombotic symptoms of APS include thrombocytopenia, hemolytic anemia, heart valve thickening, and neurological complications.
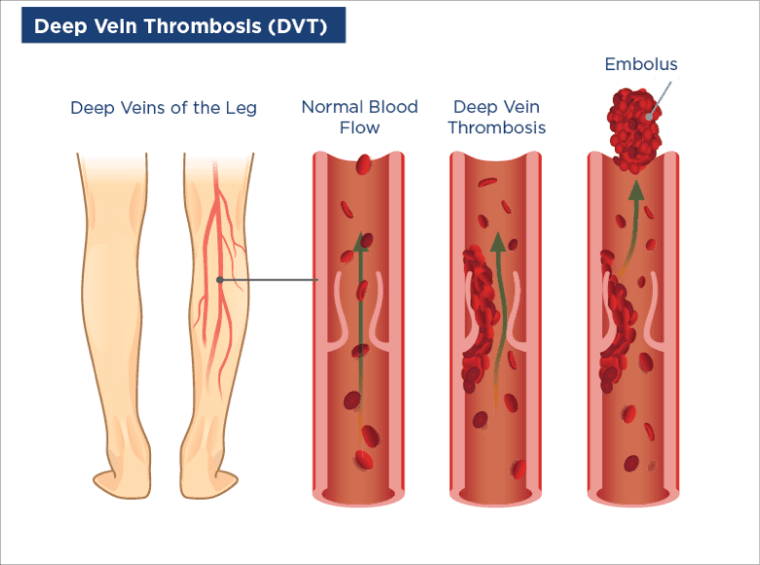
Etiology of Antiphospholipid Syndrome
There are multiple autoantibodies found in patients with APS. The cause of this antibody development remains unclear, but it is most likely the result of a complex interaction of environmental factors and genetics. In some patients, the cause may be another underlying autoimmune disease, in which case the condition is defined as secondary APS. For example, 30–40% of patients with systemic lupus erythematosus (SLE) have antiphospholipid (aPL) antibodies, and patients with connective tissue disease may test positive for such antibodies.⁴ Certain types of infection and medications are the main environmental factors associated with APS, but in many cases the cause is unknown. In the absence of other related disease, the APS is referred to as primary APS.
There are multiple autoantibodies found in patients with APS.
Epidemiology of Antiphospholipid Syndrome
Antiphospholipid antibodies have been reported to occur in about 5% of the general population.⁵ However, the incidence of APS is much lower. APS has been reported at 2–5 new cases per 100,000 individuals per year, while the estimated prevalence is 40–50 cases per 100,000 population.⁶⁻⁷
Antiphospholipid Syndrome Risk Factors
Patients with another autoimmune disease are at particular risk of APS. Women are more prone to autoimmune disease than men and, consistent with this, the incidence of APS is higher in women than men. Infections such as syphilis, human immunodeficiency virus (HIV), cytomegalovirus, hepatitis B and C, and certain bacterial infections are associated with a higher risk of APS. For those with a family history of APS, the risk of developing the condition is higher than in the general population. Medications that have been linked with the presence of aPL, although not necessarily causing drug-induced APS, include chlorpromazine, amoxicillin, phenytoin, chlorothiazide, propranolol, oral contraceptives, quinine, alpha-interferon, and infliximab. Certain cancers have also been associated with APS development.⁴
There is a higher risk of recurrent thrombosis in those with a history of prior thrombotic events, having combined arterial and venous thrombosis, previously treated with low molecular weight heparin (LMWH), or who are receiving immunosuppressant therapy.⁸
Diagnostics for Antiphospholid Syndrome
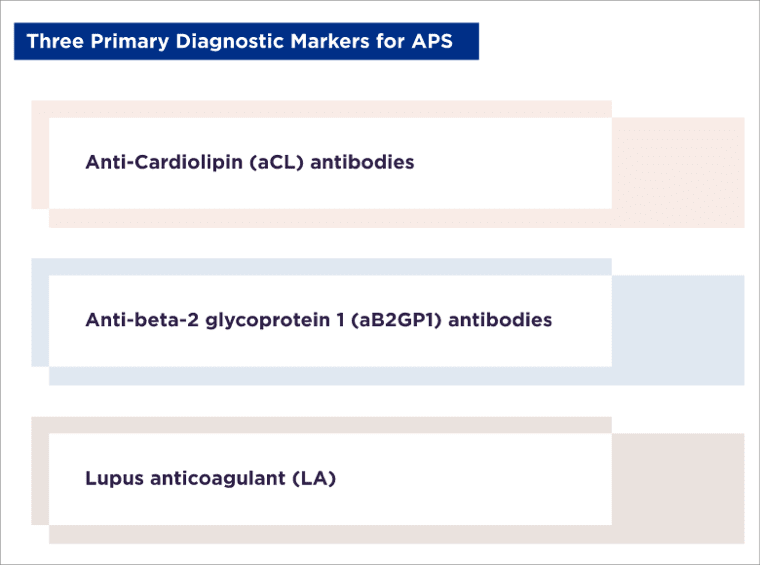
The diagnosis of APS may be based on clinical symptoms of thrombosis or pregnancy complications, or it may include the identification of certain antibodies in the blood through laboratory tests.
There are three primary diagnostic markers for APS. Two of these are aPL antibodies: anticardiolipin (aCL) antibodies and anti-beta-2 glycoprotein 1 (aB2GP1) antibodies. The third is lupus anticoagulant (LA), which is associated with clotting time. The marker type, the presence of multiple versus single markers, their titer and their persistence of over time, define an "aPL profile." A high-risk aPL profile is associated with an elevated risk of thrombosis and pregnancy complications.³
Antibodies other than aCL and aB2GP1 have also been associated with APS and may be useful diagnostic indicators. These include antiphosphatidylserine (aPhS), antiprothrombin (aPT), aPhS/PT complex and aB2GP1 immunoglobulin A (IgA) and aB2GP1-Domain 1 (D1) antibodies. Positive titers for aPhS have been reported in 70% of patients with thrombotic or obstetric problems in whom APS was confirmed. Many of these patients also have positive titers for aCL and aB2GP1. Similarly, among obstetric APS patients, aPhS positivity was identified in 88% of those who were aPL- and/or LA-negative versus 73% of those who were aPL- and/or LA-positive.¹
aPhS, aPhS/PT and aB2GP1 IgG and IgM isotypes are all associated with an increased risk of thrombosis.⁹ The evidence for IgA isotype is less clear, but it may be a relevant marker to test for in patients who are seronegative for standard APS antibodies.
Patients can have APS without a positive aPL or LA titer (seronegative APS). Diagnosis is then based on clinical symptoms and the absence of other disease, which could account for those symptoms. For example, in a study in women with a history of obstetric APS, only 24.3% were positive for at least one aPL. aPhS/PT and aCL positivity were most common, and in some patients aPhS/PT was the only aPL identified.²
A scoring system, the Global Antiphospholipid Syndrome Score (GAPSS), has been developed to measure the probability of thrombosis in patients based on their aPL titer and other cardiovascular risk factors. The higher the score, the greater the risk. Patients with a score of ≥16 are 6 times more likely to experience thrombosis.¹⁰
Treatment for Antiphospholipid Syndrome
For those with APS, whether treatment is required will depend on the aPL profile, symptoms, and clinical profile. Generally, treatment is prophylactic to prevent thrombosis, and will continue in the long term, perhaps for life.
From a review of published literature, the European League Against Rheumatism (EULAR) has developed a series of recommendations for the management of APS.3 Patients can be classified into risk categories according to aPL profile. High risk is the presence, on ≥2 occasions ≥12 weeks apart, of:
- LA
- A double combination of any of LA, aCL antibody or aB2GP1 antibody
- The triple combination of LA, aCL antibody and aB2GP1 antibody
- Persistently high aPL titers
The high-risk profile is associated with elevated risk of thrombosis and obstetric APS.
Alongside any drug treatment, risk should be managed by screening for cardiovascular and venous thrombosis risk factors, educating patients about compliance with treatment programs, and providing lifestyle counselling.
Treatment for Thrombotic APS
Prophylactic low-dose aspirin is recommended for those with a high-risk aPL profile but no symptoms, as well as in those with SLE with no history of APS symptoms. Depending on risk/benefit analysis, it may also be prescribed for non-pregnant women with a history of obstetric APS. For these types of patients, this daily treatment may be required for life.
In patients with definite APS and a first venous thrombosis, the recommended treatment (after heparin therapy) is a vitamin K antagonist (VKA)—most commonly warfarin—with a target international normalized ratio (INR) of 2–3. In those with definite APS and first arterial thrombosis, VKA is recommended over low-dose aspirin but, depending on the bleeding and recurrent thrombosis risk, low-dose aspirin may be added. If there is recurrent thrombosis (arterial or venous), low-dose aspirin can be added, the INR for anticoagulation can be increased to 3–4 or the anticoagulation can be switched to LMWH.
Treatment for Obstetric APS

In women with a high-risk aPL profile but no history of thrombosis or obstetric APS, low-dose aspirin should be considered during pregnancy. For those with a history of obstetric APS, low-dose aspirin is recommended during pregnancy, with heparin at prophylactic dosages depending on the clinical history. This treatment combination is also recommended during pregnancy in women with a history of thrombosis.
Where pregnancy complications are persistent, despite low-dose aspirin and heparin (increased to a therapeutic dosage), then hydroxychloroquine, low-dose prednisolone (in the first trimester) or intravenous immunoglobulin may be appropriate.³
Scientific materials
References
- Cuadrado MJ. Utility of alternative markers in the diagnosis of antiphospholipid syndrome. In: IV Symposium on autoimmunity. Present and future in the diagnosis and treatment of autoimmune disease. Santiago, Chile: Grifols, S.A.; 2018. pp2-7.
- Žigon P, Pirkmajer KP, Tomsic M, Kveder T, Božič B, Sodin Šemrl S, Čučnik S, Ambrožič A. Anti-phosphatidylserine/prothrombin antibodies are associated with adverse pregnancy outcomes. J Immunol Res. 2015; 2015:1-8. PUBMED link
- Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, Cuadrado MJ, Dörner T, Ferrer-Oliveras R, Hambly K, Khamashta MA, King J, Marchiori F, Meroni PL, Mosca M, Pengo V, Raio L, Ruiz-Irastorza G, Shoenfeld Y, Stojanovich L, Svenungsson E, Wahl D, Tincani A, Ward MM. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019; 78:1296-304. PUBMED link
- Willis R and Pierangeli SS. Pathophysiology of the antiphospholipid antibody syndrome. Autoimmun Highlights. 2011; 2:35-52. PUBMED link
- Koniari I, Siminelakis SN, Baikoussis NG, Papadopoulos G, Goudevenos J, Apostolakis E. Antiphospholipid syndrome; its implication in cardiovascular diseases: a review. J Cardiothorac Surg. 2010; 5:101-13. PUBMED link
- Uthman I, Noureldine MHA, Ruiz-Irastorza G, Khamashta M. Management of antiphospholipid syndrome. Ann Rheum Dis. 2019; 78:155-61. PUBMED link
- Duarte-García A, Pham MM, Crowson CS, Amin S, Moder KG, Pruthi RK, Warrington KJ, Matteson EL. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019; 71:1545-52. PUBMED link
- Sanchez-Redondo J, Espinosa G, Varillas Delgado D, Cervera R. Recurrent thrombosis with direct oral anticoagulants in antiphospholipid syndrome: a systematic literature review and meta-analysis. Clin Ther. 2019; 41:1839-62. PUBMED link
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost. 2014; 111:354-64. PUBMED link
- Sciascia S, Sana G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML . The global anti-phospholipid syndrome score in primary APS. Rheumatology (Oxford). 2015; 54:134-8. PUBMED link