Autoimmune Diabetes Diagnostics
Diabetes mellitus (DM), or diabetes, is a group of metabolic diseases, including type 1 diabetes and type 2 diabetes, characterized by high blood sugar levels over a long period. This discussion covers types, symptoms, risk factors, diagnostics and treatments for diabetes.
Symptoms of Diabetes
Diabetes symptoms include frequent urination, increased thirst and hunger, and weight loss. If left untreated, diabetes has many complications, including microvascular (small blood vessel) complications, reduced attention, visual perception, and psychomotor (conscious movement) speed.
Classifying Types of Diabetes
Type 1 Diabetes
Type 1 diabetes (T1D) results from autoimmune destruction of insulin-producing beta-cells in the pancreas. The subsequent lack of insulin leads to increased blood and urinary levels of glucose.¹ Ketoacidosis (a build-up of acids [ketone bodies] in the blood) is the presenting feature in about one-third of cases.
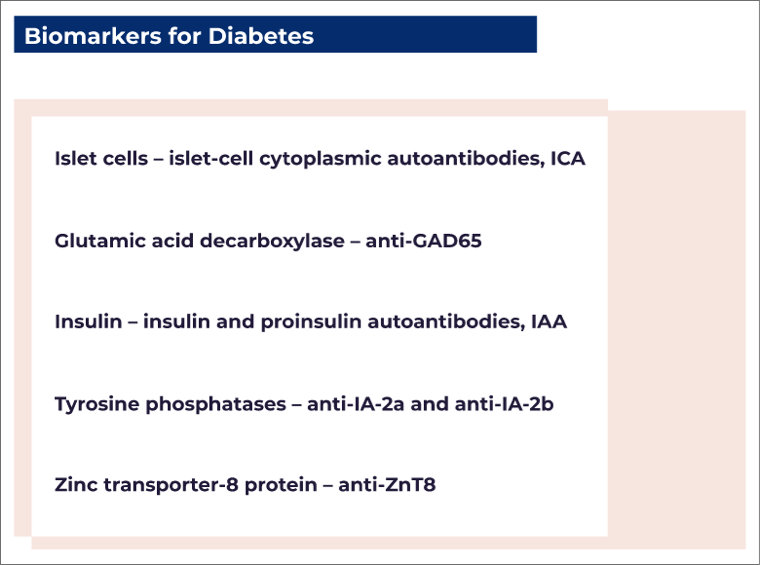
T1D accounts for 5–10% of all types of diabetes. It is defined by the presence of one or more autoimmune biomarkers (biological indicators that attack the immune system) that include autoantibodies against²:
There are different types of T1D:
- Type 1A is defined as classic autoimmune diabetes.
- Type 1B is defined as idiopathic (no known cause) or fulminant (sudden-onset) diabetes. Its features are composed of total insulin deficiency due to self-destruction of pancreatic beta-cells; there are no biomarkers of autoimmunity. About 15% of people with T1D will have type 1B diabetes. It is more common in Africans and Asians, and more frequently progresses to ketoacidosis than type 1A diabetes.
- Latent autoimmune diabetes in adults (LADA) is a slowly progressing form of autoimmune diabetes. Some doctors define this as type 1.5 diabetes, as it is considered partway between T1D and type 2 diabetes (T2D). LADA characteristics include occurrence after age 30 years, the presence of autoantibodies, and no immediate requirement for insulin; that is, insulin is usually started several months to years after LADA diagnosis. LADA requires antibody testing in all cases where an autoimmune cause is suspected. A positive antibody test provides valuable predictive (prognostic) information. It will suggest if a person is likely to have a poor response to oral antidiabetics. It will also indicate if someone has an increased risk of ketoacidosis or an earlier need for insulin.

Etiology of Type 1 Diabetes
The development of T1D is particularly complicated. An intricate interplay occurs between environmental factors, the microbiome (beneficial microorganisms inside the body), genome (genetic composition), and immune systems. This interplay varies from person to person.¹ More simply, T1D involves environmental triggers (bacteria, diet [e.g., cows' milk, wheat proteins, vitamin D deficiency], viruses) in genetically susceptible individuals causing beta-cell destruction.⁴ As beta-cells are destroyed, several proteins are released, and autoantibodies start to be produced. Even without symptoms of T1D, the presence of these autoantibodies is highly predictive of future diabetes development. Autoantibodies may be present 10–15 years before diabetes is diagnosed.
The genetic combinations with the greatest risk of T1D are HLA-DR3 and HLA-DR4-DQ8, which are associated with a >80% risk of developing ICA. Despite enhanced genetic knowledge about T1D, most people with the disorder do not have relatives with the disease. This means that primary prevention of T1D is difficult.¹
Epidemiology of Type 1 Diabetes
Worldwide, the incidence and prevalence of T1D increases by about 2–5% each year.¹⁻² In the US, the mean overall incidence from 2001–2015 was ≈23 cases per 100,000 population in individuals aged <65 years. Altogether, the largest increases in incidence were in children aged <15 years, and especially in those aged <5 years. This suggests the involvement of non-genetic factors such as behavioral and environmental influences: diet, obesity and beta-cell stress, reduced range of gut microbes, viral infections, and vitamin D deficiency.
The incidence of T1D varies markedly according to geographic region: for example, ≥30 cases per 100,000 population in Scandinavia, to <5 cases per 100,000 in China and India.⁵
Type 1 Diabetes Risk Factors
T1D is frequently associated with swift complications, notably hypoglycemia (low blood glucose) and ketoacidosis. Severe hypoglycemia, which requires treatment help from another person, has an incidence of 16–20 per 100 person-years; the corresponding value for hypoglycemia associated with seizure or loss of consciousness is 2–8 per 100 person-years. Many with T1D have hypoglycemia unawareness and fear of hypoglycemia, which may also affect their families, friends, and caregivers. Overall, hypoglycemic events have markedly detrimental effects on cognitive function, and account for 4–10% of deaths related to T1D.¹
In children with T1D, the incidence of in-hospital treatment for ketoacidosis is up to 10 per 100 person-years; further, ketoacidosis is the cause of up to 20% of deaths related to T1D.¹
- Microvascular (small blood vessel) complications — retinopathy (disease of the retina), neuropathy (numbness or weakness in peripheral nerves), kidney disease, and effects on the heart and other tissues. Hyperglycemia (high blood glucose) is the principal cause of microvascular complications
- Macrovascular (large blood vessel) complications — atherosclerosis (fatty deposition in arterial walls) and thrombosis (blood clotting) in the brain, heart, and peripheral arteries
- Reduced neurocognitive (brain) function — reduced attention, visual perception, and psychomotor (conscious movement) speed
- Other autoimmune diseases — autoimmune gastritis (stomach inflammation), autoimmune hepatitis (liver inflammation), celiac disease, primary adrenal insufficiency, and thyroid disease. The American Diabetes Association (ADA) recommends that soon after a diagnosis of T1D, consideration should be given to testing patients for autoimmune thyroid disease and celiac disease⁶
- Hip fracture — For those with T1D, the age-specific risk of hip fracture increases > 6-fold⁶
Women rather than men with T1D have higher rates of cardiovascular (heart and blood vessel) problems and early death.
If a patient has T1DM, cardiovascular disease may reduce their lifespan by approximately 10–15 years relative to healthy individuals.¹
Diagnostics for Type 1 Diabetes
Early diagnosis of T1D is important to try to maintain as much beta-cell mass and function as possible. Indeed, the release of even small quantities of insulin may help to limit the long-term complications of poor blood glucose control. Ideally, screening tests could include a combination of⁴:
- Genetic testing
- Autoantibody assessments (e.g., ICA, anti-GAD65, IAA, anti-IA-2, anti-ZnT8)
- An insulin C-peptide test to monitor insulin secretion
- Metabolomic profiling (measurement of specific small molecules)
The ADA defines three diagnostic stages of T1D6:
- Stage 1 — Multiple autoantibodies, no impaired glucose tolerance (IGT), no impaired fasting glucose (IFG)
- Stage 2 — Multiple autoantibodies, IGT and/or IFG, fasting plasma glucose (FPG) 100–125 mg/dL, 2-h postprandial glucose (PG) 140–199 mg/dL, glycosylated hemoglobin (A1C) 5.7–6.4% or ≥10% increase
- Stage 3 — Principal symptoms (excessive urination, thirst, hunger, weight loss), and standard diabetes criteria on two separate occasions: FPG ≥126 mg/dL, or 2-h PG ≥200 mg/dL during an oral glucose tolerance test, or A1C ≥6.5%, or, in patients with hyperglycemia, random plasma glucose ≥200 mg/dL
Current diagnostic recommendations from the ADA regarding T1D are:
- Plasma glucose rather than A1C should be used to confirm the acute onset of T1D in people with symptoms of hyperglycemia
- Screening for T1D should be conducted with a panel of autoantibody testing only for first-degree family members of patients with T1D or in a clinical research trial
- Persistence of ≥2 autoantibodies predicts T1D and suggests that treatment, in a clinical research trial, may be appropriate
Autoantibodies and Other Biomarkers
More than 90% of people with newly diagnosed T1D will have detectable autoantibodies against particular beta-cell proteins. In children, the presence of two or more autoantibody types is linked with an 84% risk of developing clinical T1D by age 18 years.¹
Thus, detection of autoantibodies is useful for confirming the diagnosis of T1D, and for predicting future development of the disease (e.g., in first-degree relatives). It may also be useful for predicting the likelihood of beta-cell damage in adults with LADA. Generally, autoantibody assessments are recommended in:
- Patients with diabetes onset at age <50 years
- Patients presenting with the principal symptoms, or ketoacidosis
- Non-obese patients (body mass index <25 kg/m2)
- Patients with a family history of autoimmune diseases
Traditionally, ICA have been widely used in the T1D arena and remain the gold standard of autoantibody assessment; however, pancreatic biopsy is necessary followed by indirect immunofluorescence. Other autoantibodies are assessed by enzyme-linked immunosorbent assay. Measurement of IAA is recommended in the first 2 weeks after starting insulin therapy, to monitor for cross-reactivity; IAA are often positive in patients aged <12 years but are rarely positive in adults.
In clinical practice, the most commonly evaluated autoantibodies are anti-GAD65 (present in 70–80% of patients at diagnosis), and ICA and anti-IA-2a and anti-IA-2b (60–70%). The latter autoantibodies usually persist longer in patients' sera than do anti-GAD65 and IAA. Unlike other autoantibodies, anti-GAD65 are unaffected by age. However, they are not specific to pancreatic beta-cells and are also found in glial cells in the brain.
Anti-IA-2 develop later than anti-GAD65 and can serve as short-term markers of risk. Like IAA, anti-IA-2 are more common in children than adults.
Anti-ZnT8 (60–80%) appear later in the disease course than anti-GAD65 and anti-IA-2a and tend to become negative early, but this is not always the case. An association exists between anti-ZnT8 and age at diabetes onset, but this becomes negative as individuals age.
CD38 is a cell surface receptor considered to be a physiologic mediator of insulin secretion. Anti-CD38 antibodies have been found in up to 20% of patients with T1D and up to 20% of patients with T2D.
Effective biomarkers predicting disease progression and treatment response are not yet available. Nonetheless, research in this area is ongoing. In the future, panels of biomarker assessments might include one or more of the following²:
- Proteomic research — e.g., GAD65 deamidation, hybrid insulin peptides
- Novel autoantibodies — e.g., anti-tetraspanin-7, anti-islet cell surface antigen
- Nucleic acid biomarkers — e.g., microRNAs
- Metabolomic biomarkers — e.g., sphingolipids
Treatment for Type 1 Diabetes
Guideline-based goals for A1C in T1D patients are <7.0% (adults) and <7.5% (children). However, most patients do not attain these goals. Targets should be individualized according to factors such as other diseases, patient awareness, and resource availability.
The mainstay of T1D treatment remains basal (long-acting) plus bolus (short-acting) insulin therapy administered via pen or pump. Pramlintide, an islet-cell amyloid polypeptide stimulant, is also approved for use in T1D. Other agents, such as abatacept (a CD80 inhibitor) and teplizumab (a CD3 inhibitor), are currently undergoing research in stage 1–2 T1D.¹

Scientific materials
References
- DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018; 391:2449–62. PUBMED link
- Yi L, Swensen AC, Qian W-J. Serum biomarkers for diagnosis and prediction of type 1 diabetes. Transl Res. 2018; 201:13–25. PUBMED link
- Davis TME, Wright AD, Mehta ZM, Cull CA, Stratton IM, Bottazzo GF, Bosi E, Mackay IR, Holman RR. Islet autoantibodies in clinically diagnosed type 2 diabetes: prevalence and relationship with metabolic control (UKPDS 70). Diabetologia. 2005; 48:695–702. PUBMED link
- Van Belle TL, Coppieters KT, Von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011; 91:79–118. PUBMED link
- International Diabetes Federation. IDF Diabetes Atlas, 9th edition; Brussels, Belgium: IDF; 2019 [accessed December 2019]. Available at: https://www.diabetesatlas.org/en/. link
- Riddle, MD Matthew C., Bakris, MD, George; Blonde, MD, FACP, Lawrence; Boulton, MD, Andrew J.M.; D'Alessio, MD, David; de Groot, PhD, Mary et al. Standards of medical care in Diabetes. ADA : 2018; VOLUME 41; SUPPLEMENT 1: S1-S193. Available at: https://diabetesed.net/wp-content/uploads/2017/12/2018-ADA-Standards-of-Care.pdf. link