BLOODchip® ID CORE XT predicts phenotypes that cannot be resolved by serology by analyzing polymorphisms of 37 blood group antigens of RHCE, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright and Lutheran blood group systems.
Manage chronically transfused patients by knowing the patient’s genotype for their lifespan with cost effective predictive phenotyping. It complements serology by giving you additional antigen identification and can work through pretransfusion testing of patients with monoclonal antibody therapies with ease.
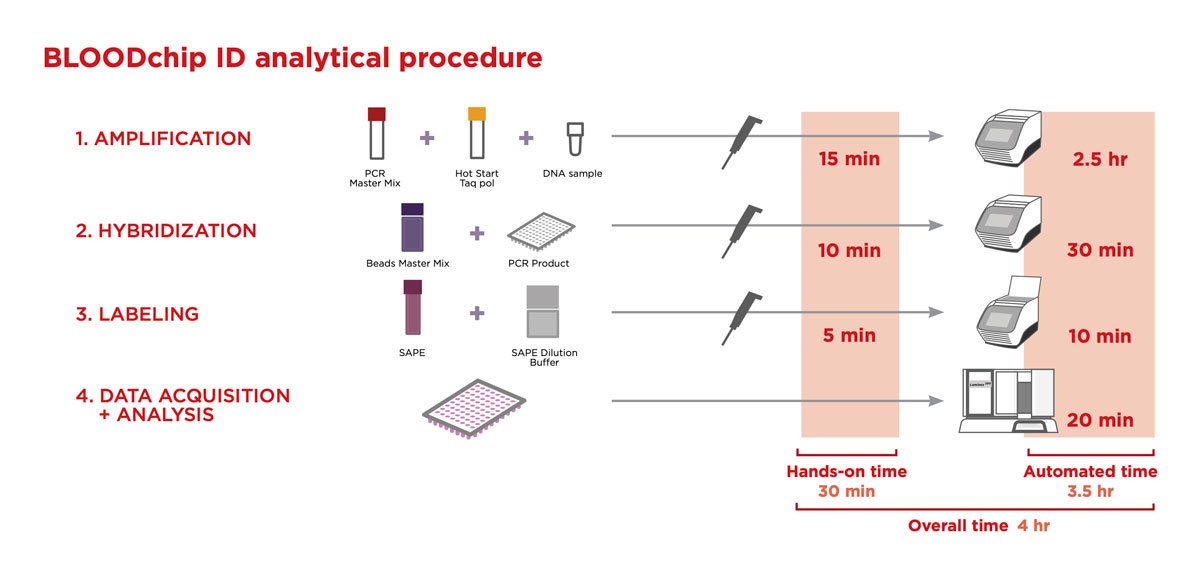
Improve management of rare antigen negative units and quickly find compatible donors for all immunized patients with this user-friendly workflow ready in 4 hours and requiring just 30 minutes of hands-on time.